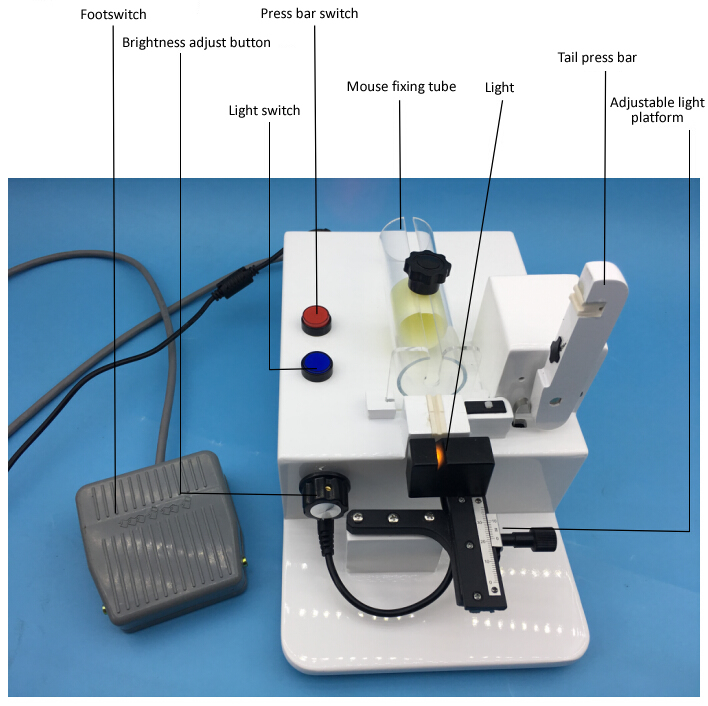
Knowlegde points of mouse blood collection
Blood collection is a must-have skill for a mouse serum supplier. What minor knowledge points should you be aware of before starting blood collection?
The circulating blood volume of mice is about 72ml/kg for blood collection. So for a 25g adult mouse, its total circulating blood volume is about 1.8ml. On the other hand, rats’ circulating blood volume is approximately 64 ml/kg for blood collection. So for a 250g adult rat, its total circulating blood volume is about 16ml.
Collect sterile blood is a prerequisite condition to centrifuge and filter qualified mouse serum.
| Circulating blood volume in laboratory animals | ||
| Blood volume (ml/kg) | ||
| Recommended mean | Range of means | |
| Mouse (25g) | 72 | 63-80 |
| Rat (250g) | 64 | 58-70 |
Around two weeks break after collecting 10% of circulating blood volume.
It is recommended that the blood collection of mice in good condition doesn’t exceed 10% of the circulating blood volume. Some studies have shown that if the volume of collecting blood is more than 15%, the experimental animals will occur shock due to blood reduction. For 25g adult mice, 10% of circulating blood volume is about 0.2ml.
Max. blood sample volumes for mouse and rat:
| Total blood volumes and recommended maximum blood sample volumes (ml) | |||||
| Total blood volumes | 7.50% | 10% | 15% | 20% | |
| Mouse (25g) | 1.8 | 0.1 | 0.2 | 0.3 | 0.4 |
| Rat (25g) | 16 | 1.2 | 1.6 | 2.4 | 3.2 |
After that, it takes around two weeks for the mice to recover their blood indicators to normal levels. The following table lists the recovery periods for different blood volumes:
| Limit volumes and recovery periods | |||
| Single sampling | Multiple sampling | ||
| Circulatory blood volume removed | Approximate recovery period | Circulatory blood volume removed in 24h | Approximate recovery period |
| 7.5% | 1 week | 7.5% | 1 week |
| 10% | 2 weeks | 10-15% | 2 weeks |
| 15% | 4 weeks | 20% | 3 weeks |
If it requires collection on multi-points, the maximum blood volume per week should not exceed 7.5% of the circulating blood volume. Besides, the maximum blood volume every two weeks should not exceed 10% of the circulating blood volume.
The difference between whole blood, plasma and serum
Whole blood
The whole blood includes all components of blood cells and plasma. When collecting whole blood, the collection tube must be added anticoagulant. Conventional anticoagulants are heparin, sodium citrate, and ethylenediamine acetate (EDTA).
Plasma
The plasma is a cell-free liquid obtained from whole blood after anticoagulation treatment and centrifugation. As well as the plasma contains fibrinogen, coagulation factors, etc.
Mouse serum
The mouse serum is a liquid collected from whole blood without anticoagulation treatment. In other words, the serum is released from coagulated blood, with no fibrinogen and coagulation factors.
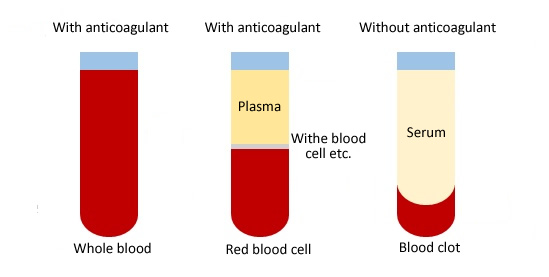
The general method for separating plasma and serum is to centrifuge at 2200-2500rpm for at least 15 minutes.
Anesthetize mice before blood collection from retro-orbital venous plexus
Before blood collection from the orbital venous plexus, mice must be generally anesthetized.
If general anesthesia is not available, local anesthesia can be applied to the eye with proparacaine or tetracaine before the blood collection operation.
The retro-orbital venous plexus method has the advantages of high success rate, low mortality of mice, fast blood flow, high volume of blood collection, and minor wounds. However, a sterile blood sample cannot be collected by this method, and the blood may be mixed with interstitial fluid and glandular secretions from the eye socket.
Suppose multiple blood collections are required to ensure wound healing in mice. In that case, there should be a 10-14 days interval between the two blood collections if misoperation or multiple blood collections of the same eye may cause some complications, such as eye bleeding, inflammation, and blindness.
Which method of multiple blood collection is better than orbital venous plexus?
From the top, submandibular vein and saphenous vein blood collections are better than the retro-orbital venous plexus method. Neither method requires anesthesia before operation. Moreover, the wound to mice is small, and the volume of blood collection is equivalent to that of the orbital venous plexus method.
What other methods for collecting blood from mice?
There are several commonly used methods from different parts of mice.
Such as: tail blood sample collection, orbital venous plexus blood collection, submandibular vein method, saphenous vein collecting method, heart blood collection etc.
Comparison of different blood collection methods:
Table 1.
| Blood sampling method | Submandibular vein | Retro orbital | Tail | Eye extraction |
| Multiple sampling | multiple, high volume | multiple, less volume | multiple, less volume | single, high volume |
| Volume/ml | 0.3-0.6 | 0.2-0.3 | 0.02-0.2 | 0.2-0.6 |
| Hemolysis | rare | rare | rare | common |
| Anesthesia | no | no | no | yes |
| Contamination | rare | rare | rare | rare |
| Difficulty level | easy | middle | easy | middle |
| Time/minute | 0.5-1 | 1-1.5 | 2-4 | 0.8-1.2 |
Table 2.
| Blood sampling method | Decollation | Cardiac puncture | Axillary artery | Saphenous vein |
| Multiple sampling | single, high volume | multiple, high volume | single, high volume | multiple, less volume |
| Volume | 0.7-1.1 | 0.5-0.6 | 0.3-0.5 | 0.03-0.2 |
| Hemolysis | common | rare | rare | rare |
| Anesthesia | yes | yes | yes | no |
| Contamination | common | no | rare | rare |
| Difficulty level | easy | hard | hard | middle |
| Time/minute | 1-1.5 | 1-3 | 2-3 | 1-2 |